Summary:
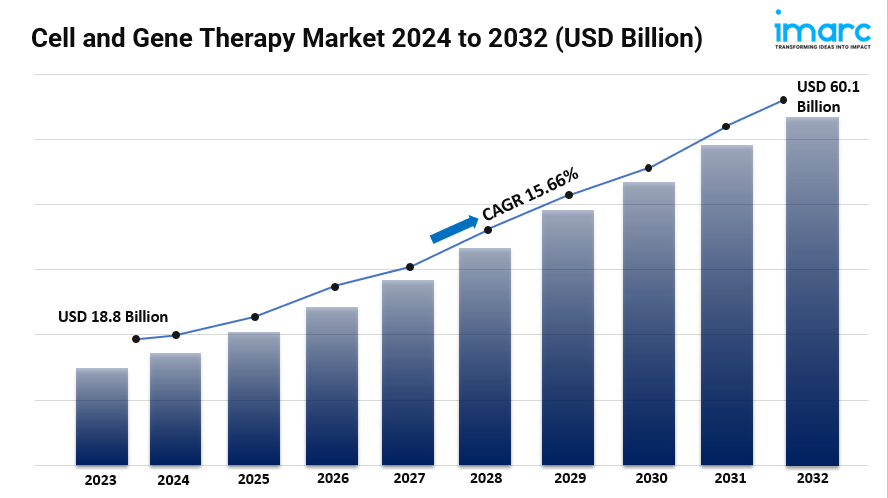
- The global cell and gene therapy market size reached USD 18.8 Billion in 2023.
- The cell and gene therapy market size is expected to reach USD 60.1 Billion by 2032, exhibiting a growth rate (CAGR) of 15.66% during 2024-2032.
- North America leads the market, accounting for the largest cell and gene therapy market share due to its advanced healthcare infrastructure.
- Based on therapy type, the market has been divided into cell therapy (stem cell (pluripotent stem cell, cancer stem cell, and adult stem cell) and non-stem cell (T cells, natural killer, and others) and gene therapy.
- Oncology disorder accounts for the majority of the market share in the indication segment owing to high prevalence of cancer.
- Based on the delivery mode, the market has been bifurcated into in-vivo and ex-vivo.
- Hospitals hold the largest share in the cell and gene therapy industry because they are primary facilities to administer complex therapies.
- The rising prevalence of chronic diseases across the globe is a primary driver of the cell and gene therapy market.
- Technological advancements and the regulatory approvals are further reshaping the cell and gene therapy market.
Industry Trends and Drivers:
- Growing Prevalence of Chronic Diseases:
The rise in chronic diseases, including cancer, cardiovascular conditions, and rare genetic disorders, is significantly boosting the demand for cell and gene therapies. Traditional treatments for these conditions, such as chemotherapy or small-molecule drugs, often fail to address the underlying causes, leading to limited long-term success. In contrast, cell and gene therapies offer a targeted, potentially curative approach by directly addressing genetic abnormalities. Furthermore, the growing number of patients diagnosed with chronic diseases is creating an urgent need for more effective treatments, particularly for conditions with high morbidity and mortality rates. Rare genetic diseases, often lacking adequate treatment options, are also gaining attention due to gene therapy's ability to target specific molecular defects.
- Regulatory Support and Accelerated Approvals:
Regulatory frameworks have become increasingly supportive of cell and gene therapies, significantly propelling market growth. Regulatory bodies are offering expedited pathways to promote the development of innovative therapies. These programs are designed to shorten the timeline from clinical trials to market authorization, allowing patients to access life-saving treatments sooner. Moreover, regulators are increasingly providing guidance on manufacturing standards and quality controls, helping companies scale production while maintaining compliance. Financial incentives, such as tax credits for clinical testing and market exclusivity for orphan drugs, further encourage investment in this space. This regulatory support is not only accelerating the approval of new therapies but also increasing investor confidence, leading to greater funding and faster innovation.
- Technological Advancements:
Technological innovations, particularly in gene editing and delivery systems, are central to the growth of the cell and gene therapy market. The development of gene-editing tools has revolutionized the field by allowing precise alterations to genetic material. These technologies have opened doors to treating previously untreatable genetic disorders by correcting defective genes at their root. Additionally, improvements in delivery mechanisms, such as viral vectors, ensure that therapeutic genes are efficiently introduced into target cells. These vectors are designed to minimize immune responses and maximize safety, leading to better patient outcomes. Moreover, the development of non-viral delivery systems, such as lipid nanoparticles, is further advancing the potential of gene therapies by addressing safety concerns linked to viral vectors. These advancements are not just enhancing efficacy but also reducing costs, as technologies become more refined and scalable.
Request for a sample copy of this report: https://www.imarcgroup.com/cell-gene-therapy-market/requestsample
Cell and Gene Therapy Market Report Segmentation:
Breakup By Therapy Type:
- Cell Therapy
- Stem Cell
- Pluripotent Stem Cell
- Cancer Stem Cell
- Adult Stem Cell
- Non-Stem Cell
- T Cells
- Natural Killer
- Others
- Stem Cell
- Gene Therapy
Based on therapy type, thee market has been divided into cell therapy (stem cell (pluripotent stem cell, cancer stem cell, and adult stem cell) and non-stem cell (T cells, natural killer, and others) and gene therapy.
Breakup By Indication:
- Cardiovascular Disease
- Oncology Disorder
- Genetic Disorder
- Infectious Disease
- Neurological Disorder
- Others
Oncology disorder dominates the market due to the high prevalence of cancer and the significant success of cell and gene therapies in treating various cancer types.
Breakup By Delivery Mode:
- In-Vivo
- Ex-Vivo
Based on the delivery mode, the market has been bifurcated into in-vivo and ex-vivo.
Breakup By End User:
- Hospitals
- Cancer Care Centers
- Pharmaceutical & Biotechnology Companies
- Others
Hospitals hold the majority of shares because they are the primary facilities for administering complex therapies that require specialized equipment and medical expertise.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America holds the leading position owing to advanced healthcare infrastructure, significant investments in research and development, and favorable regulatory frameworks supporting cell and gene therapy innovation.
Ask Analyst & Browse full report with TOC & List of Figures: https://www.imarcgroup.com/request?type=report&id=6964&flag=C
Top Cell and Gene Therapy Market Leaders:
The cell and gene therapy market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies.
Some of the key players in the market are:
- Amgen Inc.
- Biogen
- Bluebird bio, Inc.
- Bristol-Myers Squibb
- Gilead Science
- Kolon TissueGene Inc.
- Orchard Therapeutics plc.
- Pfizer Inc.
- Renova Therapeutics
- Spark Therapeutics, Inc.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Browse More Related Reports:
- Bauxite Market Size, Share, Analysis and Forecast 2024-2032
- Hybrid Adhesives & Sealants Market Size, Trends and Forecast 2024-2032
- Industrial Chocolate Market Trends, Growth and Forecast 2024-2032
- Small Modular Reactor Market Size, Analysis and Forecast 2024-2032
- Solid State Battery Market Size, Growth, Opportunity and Forecast 2024-2032
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Media Contact
Company Name: IMARC Group
Contact Person: Elena Anderson
Email:Send Email
Phone: +1-631-791-1145
Address:134 N 4th St.
City: Brooklyn
State: NY
Country: United States
Website: https://www.imarcgroup.com