
The use of multiplex assays in companion diagnostics for increasing the safety & efficacy of therapies and the advantages of multiplex assays over conventional methods are the major factors driving the growth of the multiplex assays market.
The global multiplex assays market is projected to reach $3.35 Billion in 2023 from $2.17 Billion in 2017, at CAGR of 7.5%.
Download PDF Brochure:
https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=61593314
Multiplex Assays Technologies:
1. Flow Cytometry
2. Fluorescence Detection
3. Luminescence
4. Multiplex Real-Time PCR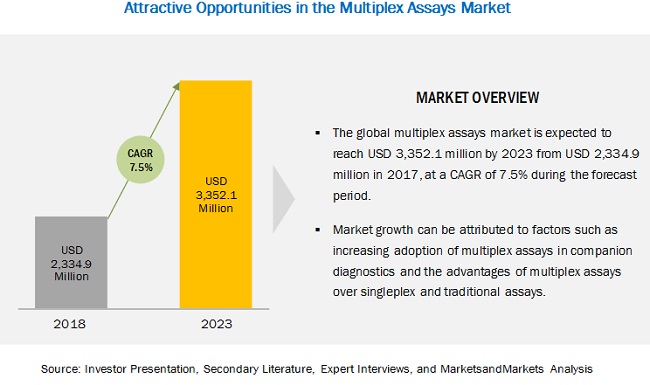
The flow cytometry segment is expected to grow at the highest CAGR in the global multiplex assays market. The high CAGR can be attributed to the wide applications of flow cytometry in detection & measurement of protein expression, RNA, cell health status (cell viability & toxicity), and characterization & identification of various cell types.
Geographical Growth and Demand Analysis:
The APAC market is projected to register the highest growth rate during the forecast period primarily due to its pharmaceutical market, which is growing at the fastest pace in the world, the availability of a large number of qualified researchers, and increasing prevalence of cancer and infectious diseases.
North America is expected to account for the largest share of this market, followed by Europe. Growth in the North American market is mainly driven by the increasing per capita healthcare expenditure and the presence of technologically advanced healthcare infrastructure in the region. Initiatives taken by different government associations are also anticipated to boost market growth in the coming years.
Request Sample Pages:
https://www.marketsandmarkets.com/requestsampleNew.asp?id=61593314
Key Players:
The key players in the global multiplex assays market are Luminex (US), Bio-Rad Laboratories (US), Becton Dickinson (US), Illumina (US), and Thermo Fisher Scientific (US).
Anti-TNF-alpha biologics are proving to be an effective treatment for autoimmune diseases, such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). With the increasing healthcare costs as well as patent expirations, the number of R&D activities focusing on the development of biosimilars have increased over the last few years. The launch of biosimilars in the market is increasing the need for the better understanding of potential differences in biosimilar immunogenicity as well as their safety and efficacy profiles as compared to their precursors.
TDM assays provide a tool for measuring the drug level of CT-P13 (Remsima and Inflectra). The Infliximab Drug Level ELISA has been tested and shown to successfully cross-react with and accurately quantify levels of CT-P13 in patient samples. This will help the study of therapeutic biologic drug concentration optimization to understand how to avoid dose toxicity.
Media Contact
Company Name: MarketsandMarkets
Contact Person: Mr. Sanjay Gupta
Email:Send Email
Phone: 18886006441
Address:630 Dundee Road Suite 430
City: Northbrook
State: IL 60062
Country: United States
Website: https://www.marketsandmarkets.com/Market-Reports/multiplex-assays-market-61593314.html
