According to a report, "In Vitro Diagnostics (IVD) Quality Control Market Analysis Report By Applications (Clinical Chemistry, Immunochemistry, Hematology, Molecular Diagnostics, Coagulation, Microbiology), By End-use, And Segment Forecasts, 2018 - 2024" , published by Grand View Research, Inc., The global IVD quality control market is expected to reach over USD 1.13 billion by 2024, The continually evolving technology-oriented changes in the diagnostics field and the growing requirement to ensure patient safety necessitate the implementation of quality assurance programs in various medical disciplines including radiology and point-of-care devices.
Key Takeaways from the report:
-
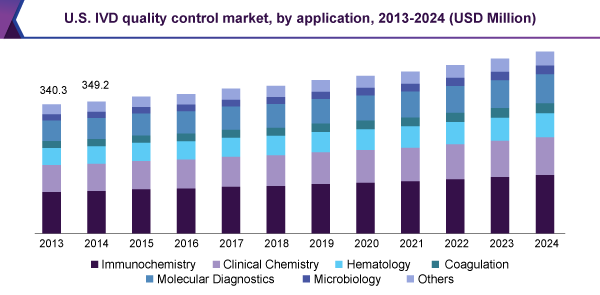
The molecular diagnostics is one of the fastest growing segments during the forecast period owing to the increasing technical complexity of molecular diagnostic testing coupled with the constant need for quality evaluation to ensure standards. The molecular diagnostics tests are of prime importance as the test outcomes enable healthcare practitioners make critical treatment decisions.
-
The hospital segment held the largest share in 2015 due to the presence of highly advanced technology-based devices, such as Next Generation Sequencing (NGS), mass spectrophotometry, and microarrays, and the rising applications of the optimized quality-control procedures
-
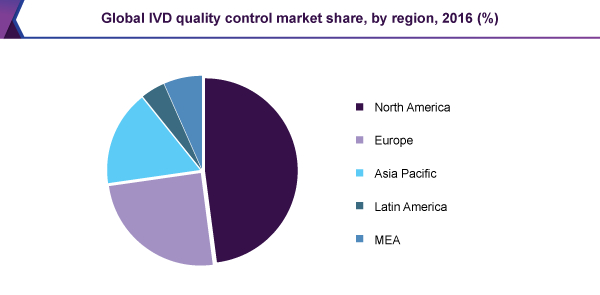
The North America contributed over 43% of the market share in 2015 attributable to the presence of over 150,000 registered diagnostics laboratories. The clinical laboratories are required to provide accurate results and maintain the accuracy standards in order to retain their license to operate.
-
Many pharmaceutical companies are implementing the new draft guidance enforced by the U.S. FDA for data integrity on current Good Manufacturing Practices (cGMP).It provides information in relation to establishing robust operating procedures and strong quality management systems, obtaining high-quality raw materials, investigating quality deviations, and maintaining reliable diagnostic laboratories
Browse More Reports in Biotechnology Industry:
Bioinformatics Services Market: Growing adoption of I.T. solutions for different studies such as cell signaling, pathway metabolism, gene-receptor interactions, and target differentiation are expected to support growth in this market over the forecast period. Owing to the benefits associated with the use of informatic solutions for laboratory protocol generation and data management as well as analysis, these solutions are projected to witness substantial demand in the coming years.
Biomarkers Market: The growing demand for companion diagnostics and the rising adoption of personalized medicine are the key factors propelling the growth of the biomarkers market. The increasing need of disease-specific biomarkers for the development of diagnostics, the increasing R&D funding, and the rising prevalence of oncology and cardiovascular-based diseases are anticipated to create significant opportunities for the market growth during the forecast period. The spiraling population and the upward trend in the adoption of sedentary lifestyles are expected to provide the industry with a huge target population base.
The patients rely on self-testing IVD devices for long-term disease management and hence it is important for such IVD devices to be checked for quality in terms of result reproducibility and validity in order to guarantee patient safety. The rising number of certified clinical laboratories offering dependable IVD-based diagnostic services directly correlates with increased patient confidence, thus driving the quality control market.
In addition to quality assessments, amendments to the regulatory framework are made intermittently to enhance the present quality standards with the main objective of safeguarding qualitative superiority of the diagnostic services rendered to patients.
In May 2016, the European Union passed an agreement to update the pre-existing regulations pertaining to IVD devices wherein the updates were in concern with raising the patient safety levels, particularly for disabled persons. Furthermore, the presence of third party quality control agencies for independent assessment of the IVD devices is expected to elevate the current safety standards, which is expected to further propel the market growth.
Grand View Research has segmented the IVD quality control market on the basis of application, type, end-use, and region:
IVD Quality Control Market Application Outlook, by Revenue (USD Million, 2013 - 2024)
-
Clinical Chemistry
-
Immunochemistry
-
Hematology
-
Molecular Diagnostics
-
Coagulation
-
Microbiology
-
Others
IVD Quality Control Market Type Outlook, by Revenue (USD Million), 2013 - 2024
-
Quality Controls
-
Plasma-based Controls
-
Serum-based Controls
-
Whole Blood-based Controls
-
Other IVD Quality Controls
-
-
Data Management
-
Quality Assurance Services
IVD Quality Control Market End-use Outlook, by Revenue (USD Million), 2013 - 2024
-
Home-care
-
Laboratory
-
Hospitals
-
Others
IVD Quality Control Market Regional Outlook, by Revenue (USD Million), 2013 - 2024
-
North America
-
U.S.
-
Canada
-
-
Europe
-
Germany
-
UK
-
-
Asia Pacific
-
Japan
-
China
-
-
Latin America
-
Brazil
-
-
MEA
-
South Africa
-
Explore the BI enabled intuitive market research database, Navigate with Grand View Compass, by Grand View Research, Inc.
About Grand View Research
Grand View Research provides syndicated as well as customized research reports and consulting services on 46 industries across 25 major countries worldwide. This U.S.-based market research and consulting company is registered in California and headquartered in San Francisco. Comprising over 425 analysts and consultants, the company adds 1200+ market research reports to its extensive database each year. Supported by an interactive market intelligence platform, the team at Grand View Research guides Fortune 500 companies and prominent academic institutes in comprehending the global and regional business environment and carefully identifying future opportunities.
For more information: www.grandviewresearch.com
Media Contact
Company Name: Grand View Research, Inc.
Contact Person: Sherry James, Corporate Sales Specialist - U.S.A.
Email:Send Email
Phone: 1-415-349-0058, Toll Free: 1-888-202-9519
Address:201, Spear Street, 1100
City: San Francisco
State: California
Country: Switzerland
Website: www.grandviewresearch.com/industry-analysis/in-vitro-diagnostics-ivd-quality-control-market
