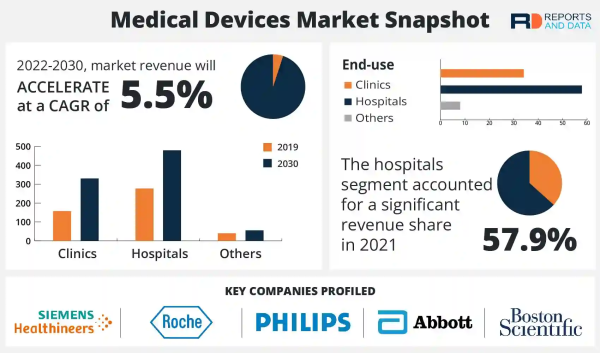
The medical devices market size is expected to reach USD 864.49 Billion in 2030 and register a revenue CAGR of 5.5% over the forecast period, according to the latest report by Reports and Data. Integration of Augmented Reality (AR) in biotechnology is driving market revenue growth. AR devices help surgeons in operating rooms during minimally invasive and non-life-threatening surgeries and AR glasses are also being used by nurses to locate veins and arteries for injections. Technological advancements in AR and Virtual Reality (VR) have enhanced learning efficiency for healthcare practitioners and increased empathy by allowing patients or their families to understand the impact of a sickness or condition. It can also be utilized for surgical visualization and patient and clinician education.
Medical devices help to diagnose and treat diseases easily. Pacemakers, imaging instruments, dialysis machines, and implants are among the most well-known and commonly used medical devices. Technological advancements have made it possible to develop innovative and efficient devices, which are driving revenue growth of the market. Remote monitoring of pulse oximetry devices, for example, is a beneficial approach for clinicians to keep in touch with their patients without the burden of hospital/clinic visitations for disorders such as chronic obstructive pulmonary disease, sleep apnea, and a variety of cardiovascular ailments.
Medical technologies such as wearables are becoming more popular in the aftermath of the coronavirus outbreak. To restrict the spread of COVID-19, the necessity to diagnose, treat, and monitor patients without human interaction has intensified, leading to an increased usage of medical technologies that will allow medical practitioners to remotely treat their patients. Artificial Intelligence (AI) in human care, wearable medical equipment, remote patient monitoring devices, Electronic Health Records (EHR), and other technologies make remote monitoring possible. These medical technological solutions enable patients to be monitored in real-time, whether at home or in hospitals, which is critical in the fight against COVID-19.
Some Key Highlights From the Report
- On 19 January 2021, Johnson & Johnson Medical Devices Companies announced that its part, DePuy Synthes received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the VELYS Robotic-Assisted Solution designed for use with the ATTUNE Total Knee System. VELYS Robotic-Assisted Solution adapts to a surgeon's workflow, provides familiar control, and aids in the execution of precise bone cuts.
- The dental devices segment revenue is expected to grow at a steady rate over the forecast period. The key elements promoting their adoption are that dental diagnoses and treatments have grown more effective, less painful, and time and cost-effective. Images recorded by intraoral and extraoral scanners, for example, can now be conveniently examined on digital screens due to the development of digital radiology. Digital dental radiology technology is simple to use and produces exact, high-quality images, eliminating the time, cost, and waste involved with traditional film processing. This also helps to cut down on diagnosis and treatment time because the images are instantly available and can be shared with a practitioner or dental laboratory via the internet for speedier consultations and findings.
For More Industry Insight, Request Sample@ https://www.reportsanddata.com/download-summary-form/5089
- The market in Asia Pacific is expected to grow at a steady rate over the forecast period due to the presence of well-established healthcare infrastructure in this region. Furthermore, the region's healthcare specialists are adopting advanced diagnostic and treatment devices, which is boosting demand in the regional market. India and China are emerging pioneers in using the latest medical devices along with developing new medical devices.
- Companies profiled in the global market report include Abbott, F. Hoffmann-La Roche Ltd, Koninklijke Philips N.V., Siemens Healthcare GmbH, Stryker Corporation, Boston Scientific Corporation, Johnson & Johnson Pvt. Ltd, Medtronic GmbH, Smith & Nephew PLC, and General Electric Company.
For this study, Reports and Data has segmented the medical devices market based on type, end-use and region:
- Type Outlook (Revenue, USD Billion; 2019–2030)
- Respiratory devices
- Cardiology devices
- Orthopedic devices
- Diagnostic imaging devices
- Endoscopy devices
- Ophthalmology devices
- Wound management
- In-vitro devices
- Dental devices
- Others
- End-use Outlook (Revenue, USD Billion; 2019–2030)
- Clinics
- Hospitals
- Others
- Regional Outlook (Revenue, USD Billion; 2019–2030)
- North America
- S.
- Canada
- Mexico
- Europe
- Germany
- K.
- France
- Italy
- Spain
- Sweden
- BENELUX
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of APAC
- Latin America
- Brazil
- Rest of LATAM
- Middle East & Africa
- Saudi Arabia
- A.E.
- South Africa
- Israel
- Rest Of MEA
- North America
Request For Custom Research @ https://www.reportsanddata.com/request-customization-form/5089
Media Contact
Company Name: Reports and Data
Contact Person: John Watson
Email:Send Email
Phone: +1-212-710-1370
Address:40 Wall St. 28th floor
City: New York City
State: NY 10005
Country: United States
Website: https://www.reportsanddata.com/press-release/global-blister-packaging-market

