The psoriatic arthritis (PsA) treatment market landscape features established therapies such as HUMIRA, OTEZLA, COSENTYX, CIMZIA, and BIMZELX. Alongside these, several promising late-stage candidates—including SOTYKTU (deucravacitinib) and ILUMYA (tildrakizumab) are advancing through clinical development. For patients who do not respond adequately to TNF inhibitor monotherapy, IL-17 inhibitors are generally preferred, while IL-12/23 inhibitors are considered for those with coexisting inflammatory bowel disease or who prefer less frequent dosing. Key companies shaping the PsA market include UCB Biopharma, Sun Pharmaceutical, Bristol Myers Squibb, and Affibody AB, among others.
DelveInsight's "Psoriatic Arthritis Market Insights, Epidemiology, and Market Forecast-2034" report delivers an in-depth understanding of Psoriatic Arthritis, historical and forecasted epidemiology as well as the Psoriatic Arthritis market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom), and Japan.
The Psoriatic Arthritis market report provides current treatment practices, emerging drugs, the market share of the individual therapies, and the current and forecasted Psoriatic Arthritis market size from 2020 to 2034, segmented by seven major markets. The Report also covers current Psoriatic Arthritis treatment practice/algorithm, market drivers, market barriers, and unmet medical needs to curate the best opportunities and assesses the underlying potential of the Psoriatic Arthritis market.
Request for a Free Sample Report @ https://www.delveinsight.com/report-store/psoriatic-arthritis-market
Some facts of the Psoriatic Arthritis Market Report are:
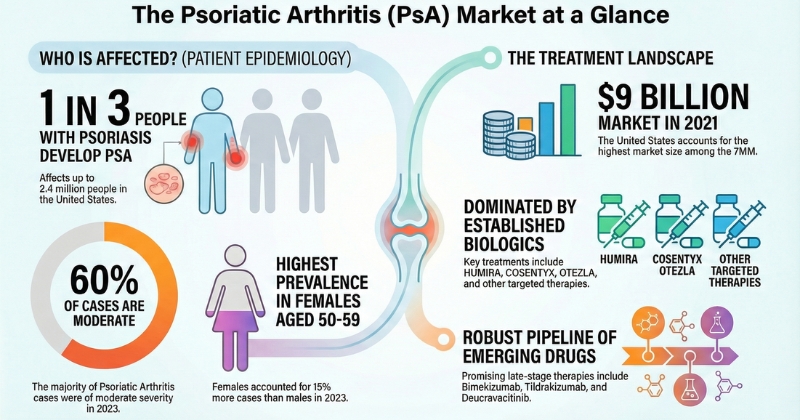
- According to DelveInsight, Psoriatic Arthritis market size was USD 9 Billion in 2021.
- Leading Psoriatic Arthritis companies working in the market are UCB Biopharma, Sun Pharmaceutical Industries Limited, BMS, Affibody AB, Janssen Biotech, Amgen, Eli Lilly and Company, Teva Pharma, Boehringer Ingelheim, AbbVie, Roche, Ampio Pharmaceuticals, Antares Pharma, Currax Pharmaceuticals, GlaxoSmithKline, Bayer, Sanofi, Astrazeneca, Johnson & Johnson Services, Pfizer and others.
- Key Psoriatic Arthritis Therapies expected to launch in the market are imekizumab (UCB Biopharma), tildrakizumab (Sun Pharmaceutical), deucravacitinib (BMS), izokibep (Affibody AB) and others.
- High Prevalence of Obesity, Growing Research Activities, and Increase Incidence of Psoriatic Arthritis are some of the factors driving the Psoriatic Arthritis market.
- The current treatment landscape includes medications such as HUMIRA (adalimumab), OTEZLA (apremilast), COSENTYX (secukinumab), CIMZIA (certolizumab pegol), and BIMZELX (bimekizumab), among others.
- In January 2026, Eli Lilly and Company (NYSE: LLY) today announced positive topline results from the novel TOGETHER-PsA open-label Phase 3b trial evaluating the concomitant use of Taltz (ixekizumab) and Zepbound (tirzepatide) compared to Taltz alone in adults with active psoriatic arthritis (PsA) and obesity or overweight with at least one weight-related condition. At 36 weeks, treatment with concomitant Taltz and Zepbound met the primary and all key secondary endpoints for superiority to Taltz monotherapy. TOGETHER-PsA is the first controlled study to evaluate an incretin therapy used with a PsA biologic. An estimated 65% of adults with PsA in the U.S. also have obesity (BMI ≥30 kg/m²) or overweight (BMI 27-29.9 kg/m²) with at least one additional weight-related comorbidity, highlighting a need for integrated treatment approaches that address the full burden of their diseases.
- In September 2025, Johnson & Johnson (NYSE: JNJ) today announced that the U.S. Food and Drug Administration (FDA) has approved TREMFYA® (guselkumab) for the treatment of children six years and older who also weigh at least 40 kg with moderate to severe plaque psoriasis (PsO), who are candidates for systemic therapy or phototherapy, or active psoriatic arthritis (PsA). This milestone makes TREMFYA® the first and only IL-23 inhibitor approved for these pediatric indications and builds on the initial FDA approvals in adults living with moderate to severe plaque PsO in 2017 and active PsA in 2020.
- In March 2025, Celltrion officially launched STEQEYMA® (ustekinumab-stba) in the U.S. following its FDA approval in December 2024. STEQEYMA, a biosimilar to STELARA® (ustekinumab), is approved for the same indications, providing consistent treatment options for both patients and healthcare providers. It is authorized for subcutaneous or intravenous use in adult and pediatric patients with plaque psoriasis and psoriatic arthritis, and in adult patients with Crohn's disease and ulcerative colitis.
- In October 2024, Dong-A ST received FDA approval for Imuldosa (ustekinumab-srlf/DMB-3115), another biosimilar to Stelara, for treating autoimmune diseases such as plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. Stelara, developed by Janssen Biotech, recorded $10.86 billion in global sales in 2023. Imuldosa offers a more cost-effective biosimilar alternative for long-term autoimmune therapy.
- On September 23, 2024, UCB announced FDA approval for BIMZELX (bimekizumab-bkzx) for the treatment of active psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis. BIMZELX is now the first and only dual IL-17A and IL-17F inhibitor approved in the U.S. for these chronic immune-mediated inflammatory conditions.
Psoriatic Arthritis Overview
Psoriatic Arthritis is a chronic inflammatory condition that affects individuals with psoriasis, leading to joint pain, stiffness, and swelling. Psoriatic Arthritis symptoms typically include fatigue, swollen fingers or toes, and reduced range of motion, which can significantly impact daily activities. Psoriatic Arthritis diagnosis involves a combination of physical examination, medical history, imaging tests, and sometimes blood tests to rule out other types of arthritis.
Psoriatic Arthritis causes are not fully understood, but genetic, immunological, and environmental factors play a crucial role. Psoriatic Arthritis treatment options include nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), and biologics to manage inflammation and prevent joint damage. Psoriatic Arthritis management requires a multidisciplinary approach including rheumatologists, dermatologists, and physical therapists for comprehensive care.
Psoriatic Arthritis prognosis varies among individuals, but early intervention greatly improves quality of life. Psoriatic Arthritis flare-ups can be triggered by stress, infections, or injury, so lifestyle modifications are important. Psoriatic Arthritis diet recommendations often focus on anti-inflammatory foods to help ease symptoms.
Understanding Psoriatic Arthritis risk factors, such as family history and existing psoriasis, can aid in earlier detection and intervention. Stay informed and consult healthcare professionals for personalized Psoriatic Arthritis care strategies.
Learn more about Psoriatic Arthritis treatment algorithms in different geographies, and patient journeys. Contact to receive a sample @ https://www.delveinsight.com/sample-request/psoriatic-arthritis-market
Psoriatic Arthritis Market Outlook
The Psoriatic Arthritis market outlook of the report helps to build a detailed comprehension of the historical, current, and forecasted Psoriatic Arthritis market trends by analyzing the impact of current Psoriatic Arthritis therapies on the market and unmet needs, and drivers, barriers, and demand for better technology.
This segment gives a thorough detail of the Psoriatic Arthritis market trend of each marketed drug and late-stage pipeline therapy by evaluating their impact based on the annual cost of therapy, inclusion and exclusion criteria, mechanism of action, compliance rate, growing need of the market, increasing patient pool, covered patient segment, expected launch year, competition with other therapies, brand value, their impact on the market and view of the key opinion leaders. The calculated Psoriatic Arthritis market data are presented with relevant tables and graphs to give a clear view of the market at first sight.
Psoriatic arthritis (PsA) is a chronic inflammatory condition that affects approximately 112 per 100,000 adults worldwide. It is more prevalent in Europe and North America than in Asia and South America. In patients with psoriasis, the prevalence of psoriatic arthritis can range from 6% to 34% in Western populations. Despite its impact, many individuals with psoriatic arthritis remain undiagnosed or receive inadequate treatment, highlighting the need for increased awareness and access to specialized care.
As per DelveInsight analysis, among the 7MM, the United States accounted for the highest number of prevalent cases of psoriatic arthritis in 2023, and these cases are expected to increase by the end of 2034 due to several key factors such as an increase in awareness and diagnosis, as well as the rapid prevalence of PsA.
Psoriatic arthritis, occurring in approximately 20% of individuals with psoriasis, is a chronic inflammatory arthritis intricately linked to psoriatic arthritis. This aggressive condition is characterized by potential significant morbidity and compromised quality of life.
Treatment approaches for psoriatic arthritis encompass a variety of strategies aimed at managing symptoms, slowing disease progression, and improving the overall quality of life. These approaches typically involve a combination of pharmacological therapies, lifestyle modifications, and, in severe cases, surgical interventions. Psoriatic arthritis treatment is highly individualized based on disease severity, response to therapy, and patient preferences, with multidisciplinary care from rheumatologists, dermatologists, and physical therapists playing a key role.
Pharmacological treatment for psoriatic arthritis primarily targets inflammation and pain relief. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and naproxen, are often the first line of defense, helping to reduce pain and inflammation. Disease-modifying antirheumatic drugs (DMARDs) like methotrexate, sulfasalazine, and leflunomide are commonly prescribed to prevent joint damage and suppress the inflammatory process. For individuals with mild-to-moderate psoriatic arthritis, oral small molecules (OSMs) like OTEZLA (apremilast) offer a convenient, non-injectable option by modulating inflammatory pathways.
Biologic agents have revolutionized the treatment landscape for psoriatic arthritis. These medications specifically inhibit key molecules in the inflammatory cascade. TNF inhibitors like HUMIRA (adalimumab), ENBREL (etanercept), and REMICADE (infliximab) are frontline biologics that target tumor necrosis factor, a crucial driver of inflammation. Patients who do not respond to TNF inhibitors may benefit from IL-17 inhibitors like COSENTYX (secukinumab) and TALTZ (ixekizumab) or IL-12/23 inhibitors such as STELARA (ustekinumab).
According to DelveInsight, the Psoriatic Arthritis market in 7MM is expected to witness a major change in the study period 2020-2034.
Psoriatic Arthritis Epidemiology
DelveInsight’s analysis shows that psoriatic arthritis (PsA) is more prevalent in females, with women accounting for about 15% more cases than men in 2023. Most patients present with moderate disease severity, representing nearly 60% of cases, followed by severe and mild forms. In the United States, PsA prevalence was highest among individuals aged 50–59 years and lowest in the 18–29 age group. Within Europe, Germany recorded the highest number of PsA cases among the EU4 and the UK, while Spain had the lowest. Additionally, about 30% of people with psoriasis develop PsA, affecting up to 2.4 million Americans. Long-term data indicate that PsA prevalence increases with psoriasis duration, reaching nearly 20% overall, with higher rates in adults than children.
Explore more about Psoriatic Arthritis Epidemiology @ https://www.delveinsight.com/sample-request/psoriatic-arthritis-market
Psoriatic Arthritis Drugs Uptake
This section focuses on the uptake rate of the potential Psoriatic Arthritis drugs recently launched in the Psoriatic Arthritis market or expected to be launched in 2020-2034. The analysis covers the Psoriatic Arthritis market uptake by drugs, patient uptake by therapies, and sales of each drug.
Psoriatic Arthritis Drugs Uptake helps in understanding the drugs with the most rapid uptake and the reasons behind the maximal use of new drugs and allows the comparison of the drugs based on Psoriatic Arthritis market share and size, which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Psoriatic Arthritis Pipeline Development Activities
Bimekizumab: UCB Biopharma
Bimekizumab, developed by UCB Biopharma, is a humanized IgG1 monoclonal antibody designed to simultaneously neutralize IL-17A and IL-17F—two pivotal cytokines involved in inflammatory pathways. While IL-17A has long been recognized as a major driver of inflammation, IL-17F shares overlapping biological functions and can independently contribute to inflammatory activity. Bimekizumab’s safety and therapeutic potential are being assessed through an extensive clinical development program spanning multiple indications, including several Phase III trials focused on psoriatic arthritis.
Tildrakizumab: Sun Pharmaceutical
Tildrakizumab, from Sun Pharmaceutical, is a humanized IgG1/k monoclonal antibody that specifically targets the p19 subunit of interleukin-23 (IL-23), blocking its interaction with the IL-23 receptor and thereby reducing the downstream release of pro-inflammatory cytokines and chemokines. Marketed as ILUMYA in the United States, it is approved for adults with moderate-to-severe plaque psoriasis who are eligible for systemic therapy or phototherapy. The therapy is currently under evaluation in Phase III clinical studies for active psoriatic arthritis, including both patients previously treated with anti-TNF agents and those who are anti-TNF naïve. Additionally, Sun Pharmaceutical has submitted marketing authorization applications in Japan for tildrakizumab in moderate-to-severe psoriasis and psoriatic arthritis.
Request for a sample report to understand more about the Psoriatic Arthritis pipeline development activities @ https://www.delveinsight.com/sample-request/psoriatic-arthritis-market
Psoriatic Arthritis Therapeutics Assessment
Major key companies are working proactively in the Psoriatic Arthritis Therapeutics market to develop novel therapies which will drive the Psoriatic Arthritis treatment markets in the upcoming years are UCB Biopharma, Affibody AB, Janssen Biotech, Boehringer Ingelheim, Currax Pharmaceuticals, Sun Pharmaceutical Industries Limited (NSE: SUNPHARMA), Bristol Myers Squibb (NYSE: BMY), Amgen Inc. (NASDAQ: AMGN), Eli Lilly and Company (NYSE: LLY), Teva Pharmaceutical Industries Limited (NYSE: TEVA), AbbVie Inc. (NYSE: ABBV), Roche Holding AG (SWX: ROG), Ampio Pharmaceuticals Inc. (NYSEAMERICAN: AMPE), Antares Pharma Inc. (NASDAQ: ATRS), GlaxoSmithKline plc (LSE: GSK), Bayer AG (ETR: BAYN), Sanofi (EPA: SAN), AstraZeneca plc (LSE: AZN), Johnson & Johnson (NYSE: JNJ), Pfizer Inc. (NYSE: PFE), and others.
Learn more about the emerging Psoriatic Arthritis therapies & key companies @ https://www.delveinsight.com/sample-request/psoriatic-arthritis-market
Psoriatic Arthritis Report Key Insights
1. Psoriatic Arthritis Patient Population
2. Psoriatic Arthritis Market Size and Trends
3. Key Cross Competition in the Psoriatic Arthritis Market
4. Psoriatic Arthritis Market Dynamics (Key Drivers and Barriers)
5. Psoriatic Arthritis Market Opportunities
6. Psoriatic Arthritis Therapeutic Approaches
7. Psoriatic Arthritis Pipeline Analysis
8. Psoriatic Arthritis Current Treatment Practices/Algorithm
9. Impact of Emerging Therapies on the Psoriatic Arthritis Market
Table of Contents
1. Key Insights
2. Executive Summary
3. Psoriatic Arthritis Competitive Intelligence Analysis
4. Psoriatic Arthritis Market Overview at a Glance
5. Psoriatic Arthritis Disease Background and Overview
6. Psoriatic Arthritis Patient Journey
7. Psoriatic Arthritis Epidemiology and Patient Population
8. Psoriatic Arthritis Treatment Algorithm, Current Treatment, and Medical Practices
9. Psoriatic Arthritis Unmet Needs
10. Key Endpoints of Psoriatic Arthritis Treatment
11. Psoriatic Arthritis Marketed Products
12. Psoriatic Arthritis Emerging Therapies
13. Psoriatic Arthritis Seven Major Market Analysis
14. Attribute Analysis
15. Psoriatic Arthritis Market Outlook (7 major markets)
16. Psoriatic Arthritis Access and Reimbursement Overview
17. KOL Views on the Psoriatic Arthritis Market
18. Psoriatic Arthritis Market Drivers
19. Psoriatic Arthritis Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
About DelveInsight
DelveInsight is a leading Life Science market research and business consulting company recognized for its off-the-shelf syndicated market research reports and customized solutions to firms in the healthcare sector.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email:Send Email
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting/conference-coverage-services