In the field of solid-phase peptide synthesis, the completeness of each chemical reaction is crucial for determining the purity and sequence accuracy of the final product. The synthesis process follows a cyclic procedure of “deprotection–washing–coupling–washing” for stepwise chain elongation. Even a minor deficiency in the coupling efficiency of a single amino acid can lead to the accumulation of impurities such as deleted peptides, making subsequent separation and purification extremely challenging. Therefore, it is essential to monitor key steps including deprotection and coupling to confirm reaction completion. Owing to their operational simplicity, rapid response, and cost-effectiveness, colorimetric methods have become indispensable detection tools. These methods provide specific color changes that visually indicate the presence or absence of free amino groups on the resin, offering critical quality control checkpoints during synthesis. This article systematically elaborates on the chemical principles, reagent preparation, and practical considerations of several classic colorimetric reagents, providing technical guidance for peptide synthesis experiments.
1. NinhydrinTest (Kaiser Test)
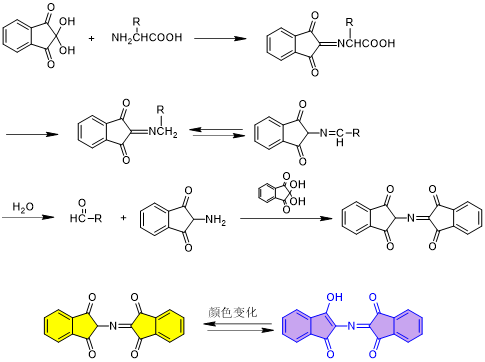
The Ninhydrin Test, proposed by Ralph Kaiser et al. and also known as the Kaiser Test, is the most widely used technique for detecting primary amines (-NH₂) and is employed to monitor the presence of free amino groups during peptide synthesis. Its core mechanism involves treating a small sample of peptide-resin with the test reagents. Ninhydrin reacts with α-amino groups (e.g., the N-terminal amine of the peptide chain) to form a characteristic blue-purple product—Ruhemann's Purple—with color intensity proportional to the amount of free amino groups present.
Note: The Kaiser Test does not detect secondary amines (e.g., proline) or amino groups lacking an α-hydrogen (e.g., diaminoisobutyric acid, α-methyl leucine, and other α,α-disubstituted amino acids).
1.1 Reagent Preparation
Three stock solutions must be prepared and mixed immediately before use. Recipes are as follows:
- Solution A:Weigh 5.0 g of ninhydrin and dilute to 100 mL with absolute ethanol. Store protected from light at room temperature. Valid for 1 month.
- Solution B:Weigh 80.0 g of phenol, add 20 mL of absolute ethanol, and dissolve completely (gentle heating may be applied to accelerate dissolution). Store protected from light at room temperature. Valid for 2 weeks. (Phenol oxidizes readily; prepare fresh before use.)
Safety Note: Phenol is highly corrosive and can be absorbed through the skin. Operate in a fume hood wearing nitrile gloves and safety goggles.
- Solution C: Add 2 mL of 0.001 M aqueous potassium cyanide (KCN) solution to 98 mL of redistilled pyridine.
Critical Hazard Alert: Potassium cyanide is extremely toxic—lethal dose is only 50–100 mg. It can be absorbed through skin, inhalation, or ingestion. Contact with acid releases lethal hydrogen cyanide gas. Prepare in a dedicated fume hood wearing a respirator, chemical-resistant suit, and nitrile gloves. Collect waste separately as highly toxic material and never allow contact with acids.
Note: KCN acts as a catalyst in the color reaction. If KCN is unavailable, it may be omitted. Additionally, during pyridine redistribution, adding a small amount of ninhydrin can help remove impurities that cause discoloration.
1.2 Testing Procedure
Transfer a small amount of washed resin into a test tube using a capillary tube. Add 1–2 drops of each of the three prepared solutions. The mixture will appear light yellow. Heat at 100–120°C for 2–3 minutes and observe the color development.
- If the solution remains yellow and the resin becomes transparent, the coupling reaction is complete.
- If the solution or resin turns blue, free amino groups are present, indicating either incomplete coupling or successful deprotection.
Important Notes:
- Using too much resin may affect the results.
- Heating time must be controlled: too short may lead to false positives (incomplete color development), while too long may cause cleavage of the Fmoc group from the amino acid or the Boc group from lysine side chains, leading to false negatives.
2.Chloranil Test
The chloranil (tetrachlorobenzoquinone) colorimetric reagent is a commonly used chromogenic agent primarily for the detection of secondary amines (such as the amino group in proline), and it can also be applied to primary amines. Additionally, de-Fmoc reagents are typically secondary amines (e.g., piperidine, morpholine, dimethylamine). Therefore, the chloranil test can be used to detect peptide resins to determine whether residual secondary amines remain after de-Fmoc treatment with a secondary amine and whether they have been thoroughly washed away.
2.1 Preparation of Test Solutions
- Solution A: Add 2 mL of acetaldehyde to 98 mL of anhydrous DMF.
- Solution B:Dissolve 2 g of chloranil in 98 mL of anhydrous DMF.
Precautions: Chloranil is irritating. Gloves and safety goggles must be worn during handling. Direct skin contact or inhalation of powder is strictly prohibited. Maintain an anhydrous environment during preparation, as moisture may interfere with the detection reaction and lead to inaccurate results.
2.2 Testing Procedure
Using a capillary tube, transfer a small amount of washed resin into a test tube. Add two drops each of Solution A and Solution B. Mix thoroughly and let stand at room temperature for 5 minutes. If the resin turns bluish-green, it indicates the presence of free amino groups. If it remains colorless or pale yellow, the coupling reaction is complete.
3. Bromophenol Blue Test
The bromophenol blue test offers advantages such as simple operation, short processing time, rapid and sensitive color development, and low toxicity. The principle of this method is based on the fact that free amino groups (–NH₂) are basic and can undergo an acid-base reaction with bromophenol blue (an acidic indicator, pKa ≈ 4.0). This reaction neutralizes H⁺ in the bromophenol blue solution, causing the indicator to change from "yellow (dissociated form, HIn)" to "blue (dissociated form, In⁻)." It can be used to detect whether the coupling reaction in solid-phase peptide synthesis is complete. If the amino groups on the resin are not free (i.e., non-basic), the bromophenol blue solution will remain yellow. If free amino groups are present, their basicity will cause the bromophenol blue solution to change from yellow to blue.
3.1 Preparation of Test Solution
Weigh 1 g of bromophenol blue powder and dissolve it in a small amount of anhydrous DMF. Then add anhydrous DMF to a total volume of 100 mL and mix well. Transfer the solution to a brown bottle, label it, and store it sealed at 4°C. For use, directly take the solution and add drops to test the resin.
3.2 Testing Procedure
Using a capillary tube, transfer a small amount of washed resin into a test tube. Add an appropriate amount of bromophenol blue reagent. If the solution immediately changes from yellow to blue, it indicates the presence of exposed amino groups on the resin, suggesting either complete deprotection or incomplete coupling.
Media Contact
Company Name: Beijing Dilun Biotechnology Co., Ltd.
Email:Send Email
Country: China
Website: https://www.peptidescientific.com/
